Whether it is a cough, a cold or a fever, many over-the-counter drugs are readily available to treat an ailing child’s symptoms. As a parent, the natural inclination is to give your child whatever they need to make them feel better, but what happens when presumably safe drugs turn out to have a deadly effect?
A Tragic Loss
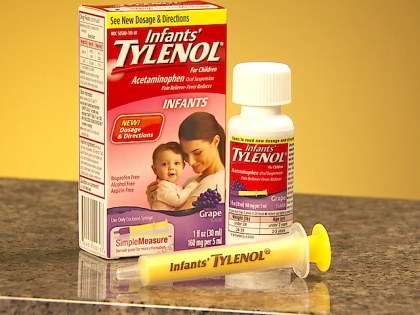
When two-year-old River Moore developed a fever, his mother gave him a dose of Very Berry Strawberry Children’s Tylenol, according to the Montgomery News. A mere two hours later, unexpected tragedy struck when the River Moore’s liver failed, and he died in the hospital.
The Resulting Lawsuit
Daniel and Katy Moore have filed suit against Johnson & Johnson, blaming the medication for their son’s death. The lawsuit includes Johnson & Johnson, McNeil-PPC and Costco Wholesale Corp, all of which the Moores allege participated in a “phantom recall” of defective products in 2010. The lawsuit claims that all companies and executives involved knew the defects of the medications but neglected to make the public aware of the potentially dangerous effects and instead issued a “stealth” recall to avoid publicity. The Moores seek compensatory and punitive damages from the defendants for the loss of their son.
McNeil Issues Recall of Children’s Tylenol
According to the McNeil Product Recall website, a recall of certain Infants’ and Children’s Tylenol was issued on April 30, 2010. In their statement, McNeil explained that the recall was voluntary because some of the products may not meet quality standards. As stated in the report, “the potential for serious medical events is remote” and “this recall is not being undertaken on the basis of adverse medical events.” The short statement also provides contact information but little else about the potential dangers of using the recalled drugs.
A May 2010 article in The New York Times provides some additional insight into the McNeil recall. According to The New York Times, the McNeil recall came directly after receiving citations for manufacturing violations found during a routine FDA inspection of their Fort Washington, PA facility. Interestingly, the violations cited included failure to thoroughly investigate customer complaints.
According to a story published in USA Today, McNeil suspended all production at the Fort Washington facility immediately following the recall. Additionally, the April recall was already the company’s fifth recall in eight months. In January 2010, McNeil was forced to recall several hundred lots of adult and children’s products after receiving reports of moldy smells emanating from products made at a plant in Puerto Rico.
McNeil’s history of poor manufacturing standards and multiple recalls does not shed a favorable light on the company. Hopefully, the Moore family will receive compensation for their terrible loss. Although nothing can give them back their son, their loss may have brought enough publicity to the issue that other would-be victims of McNeil pharmaceuticals can avoid the same tragedy.
Contact Us
When we purchase over-the-counter medication, we do so with the assumption that the drug has been properly manufactured and is safe to use. When a supposedly safe drug results in the loss of a child, there is serious problem that needs to be addressed.
If you or a loved one has been injured by defective drugs manufactured by McNeil or another company, you may be entitled to compensation. Remember, the time to file a claim is limited. Contact our caring attorneys today to schedule your free consultation.


